Compliance Recap | July 2019
July was a busy month in the employee benefits world.
The Internal Revenue Service (IRS) released a notice that expands the list of preventive care benefits a high deductible health plan can provide without a deductible or with a deductible below the annual minimum deductible. The IRS also released the indexed affordability percentage for plan years beginning in 2020.
The U.S. Preventive Services Task Force (USPSTF) published an “A” rating final recommendation. The Department of Health and Human Services (HHS) released an update to the notice requirements for plans using the HHS-administered federal external review process.
A U.S. District Court upheld the 2018 short-term, limited-duration insurance final rule. The Third Circuit Court of Appeals affirmed a federal district court’s preliminary injunction regarding contraceptive coverage exemptions.
The Department of Labor (DOL) released an advisory opinion regarding association health plans (AHPs) and multiple employer welfare arrangements (MEWAs). The Fifth Circuit Court of Appeals held oral arguments for the case challenging the ACA’s constitutionality.
HHS and the Food and Drug Administration (FDA) published a Safe Importation Action Plan regarding potential drug importation from other countries.
IRS Expands Benefits That Can Be Provided Before HDHP Annual Minimum Deductible Is Met
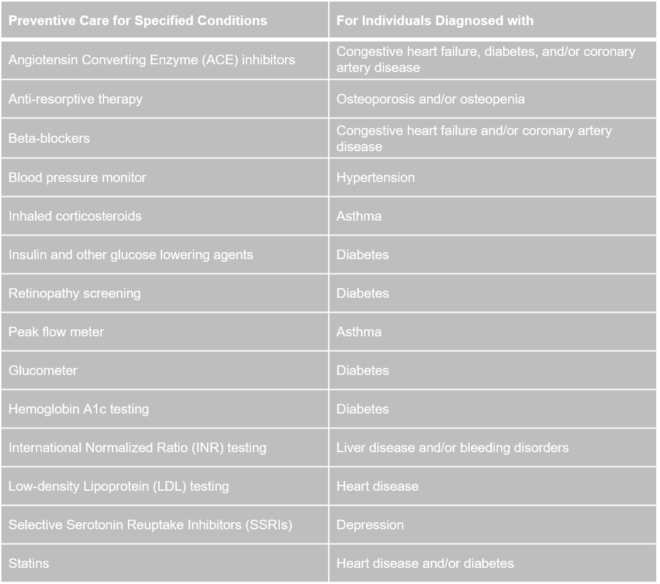
The Internal Revenue Service (IRS) released a notice, effective on July 17, 2019, that expanded the list of preventive care benefits that a high deductible health plan (HDHP) can provide without a deductible or with a deductible below the annual minimum deductible.
The services and items listed above are treated as preventive care:
- only when prescribed to treat a person diagnosed with the associated chronic condition listed in the table’s second column, and
- only when prescribed for the purpose of preventing the chronic condition’s exacerbation or a secondary condition’s development.
Read more about the expanded list of preventive care benefits.
IRS Releases the Indexed 2020 Affordability Percentage
The Internal Revenue Service (IRS) released the indexed affordability percentage of 9.78% for plan years beginning in 2020. An employer uses the affordability percentage to determine whether it has offered affordable coverage under the Patient Protection and Affordable Care Act’s employer shared responsibility provisions to avoid Penalty B.
USPSTF Issues a Final Recommendation Giving PrEP an “A” Rating
The U.S. Preventive Services Task Force (USPSTF) published a final recommendation that gives an “A” rating to preexposure prophylaxis (PrEP) treatment. This means that the USPSTF recommends offering PrEP with effective antiretroviral therapy to people at high risk of HIV acquisition.
Group health plans and insurers subject to the preventive services coverage mandate must provide coverage for evidence-based items or services with an A or B rating recommended by the USPSTF without imposing copayments, coinsurance, deductibles, or other cost-sharing requirements when delivered by in-network providers. Group health plans and insurers subject to the preventive services coverage mandate generally must cover preventive services that are recommended by the USPSTF one year after the recommendation is issued.
HHS Releases Updated Notice Requirements for the HHS Federal External Review Process
On July 12, 2019, the Department of Health and Human Services (HHS) released updated requirements for notices that self-insured non-federal governmental health plans and health insurance issuers – using the HHS-administered federal external review process – must provide to their plan participants and beneficiaries.
District Court Upholds Short-Term Limited Duration Insurance Final Rule
As background, on August 1, 2018, the Internal Revenue Service (IRS), the Department of Health and Human Services (HHS), and the Department of Labor (DOL) (collectively, the Departments) released a final rule that amended the definition of short-term, limited-duration insurance (STLDI). HHS also released a fact sheet on the final rule. The final rule allows consumers to purchase STLDI policies that are less than 12 months in length and may be renewed for up to 36 months.
On July 18, 2019, the U.S. District Court for the District of Columbia (Court) upheld the STLDI final rule. The court found that the final rule did not exceed the regulatory authority that Congress delegated to the Departments to define STDLI as a category of insurance that is exempt from individual insurance regulations. Employers should keep apprised of potential future developments as the case may be appealed.
Read more about the STDLI final rule.
Recent Litigation on the Contraceptive Coverage Exemption Rules
As background, the Department of the Treasury (Treasury), Department of Labor (DOL), and Department of Health and Human Services (HHS) (collectively, the Departments) published two final rules on November 15, 2018, regarding contraceptive coverage exemptions, to be effective on January 14, 2019. On January 14, 2019, the U.S. District Court for the Eastern District of Pennsylvania (Pennsylvania Court) granted a nationwide preliminary injunction that prohibits the implementation of the two final rules.
On July 12, 2019, the Third Circuit Court of Appeals (appeals court) affirmed the Pennsylvania Court’s preliminary injunction that prohibits the two final rules’ enforcement nationwide. The appeals court found that, until the final rules’ legality is decided, the injunction will allow states to avoid the imminent financial burden of subsidizing contraceptive services, providing funds for medical care associated with unintended pregnancies, and absorbing medical expenses that arise from decreased use of contraceptive medications for other health conditions.
The appeals court decision means that the Departments are prohibited from implementing and enforcing both final rules nationwide.
Read more about the status of the ACA contraceptive coverage mandate and exemption.
DOL Releases Advisory Opinion on AHPs and MEWAs
The Department of Labor (DOL) released an advisory opinion that analyzed a large retailer’s proposed group health plan to determine that the plan would be an association health plan (AHP) and a multiple employer welfare arrangement (MEWA) under ERISA. Although the advisory opinion can only be relied on by the retailer who requested it, the opinion gives employers an overview of the criteria that the DOL reviews when determining whether a plan fits the AHP definition that existed before the DOL’s 2018 AHP final rule. The opinion also provides a summary of the criteria that the DOL reviews when determining whether an arrangement is a MEWA.
Read more about AHPs.
Status of Court Case Challenging ACA Constitutionality
As background, in February 2018, twenty states filed a lawsuit asking the U.S. District Court for the Northern District of Texas (Court) to strike down the Patient Protection and Affordable Care Act (ACA) entirely. The lawsuit came after the U.S. Congress passed the Tax Cuts and Jobs Act in December 2017 that reduced the individual mandate penalty to $0, starting in 2019.
On December 14, 2018, the Court issued a declaratory order that the individual mandate is unconstitutional and that the rest of the ACA is unconstitutional. The Court granted a stay of its December 2018 order, which prohibits the order from taking effect while it is being appealed in the Fifth Circuit Court of Appeals (appeals court).
On July 3, 2019, the Department of Justice filed its supplemental brief to assert that the court decision striking down the ACA should not apply beyond the 18 plaintiff states. On July 9, 2019, the appeals court held oral arguments.
HHS and FDA Release Safe Importation Action Plan
The Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) issued a Safe Importation Action Plan that overviews two pathways that could permit drug importation from foreign countries. HHS also issued a press release.
Under the first pathway, HHS would propose rules to allow states, wholesalers, and pharmacists to submit demonstration project plans designed to import Canadian-approved drugs that are versions of FDA-approved drugs; meet certain conditions such as drug quality, record keeping, testing, and protection against counterfeiting; and significantly reduce consumer drug cost.
Under the second pathway, manufacturers of FDA-approved drugs could import versions of FDA-approved drugs that they sell in foreign countries if they establish with the FDA that the foreign version is the same as the U.S. version.
Question of the Month
- What if a plan sponsor fails to file or pay the PCORI fee?
- Although the PCORI statute and its regulations do not include a specific penalty for failure to report or pay the PCORI fee, the plan sponsor may be subject to penalties for failure to file a tax return because the PCORI fee is an excise tax.
A plan sponsor should consult with its attorney on how to proceed with a late filing or late payment of the PCORI fee. The PCORI regulations note that the penalties related to late filing of Form 720 or late payment of the fee may be waived or abated if the plan sponsor had reasonable cause and the failure was not due to willful neglect.
If a plan sponsor already filed Form 720 (for example, for a different excise tax), then the plan sponsor can make a correction to a previously filed Form 720 by using Form 720X.
8/1/2019